Abstract
GATA1 is a member of the GATA family of transcription factors that exerts a concentration/dependent control on the differentiation of erythroid, megakaryocytic (MK), eosinophil and mast cells. Changes of GATA1 content also controls hematopoietic stem/progenitor cell proliferation and commitment. In fact, in addition to X-linked inherited disorders of the erythroid and/or MK lineage, GATA1 mutations are found in inherited and acquired MK leukaemia and myeloproliferative disorders.
To study the regulation of the human β-globin locus, the Stamatoyannopoulos laboratory generated transgenic mice carrying human GATA1 driven by the human μLCR coupled with the β-globin promoter (hGATA1) (Li Q et al. PNAS 1997, 18;94:2444). These transgenic mice are born viable with normal phenotype and express hGATA1 at high levels in embryonic and fetal erythroblasts and barely detectable in the adult ones, raising the question whether in adult cells expression of the endogenous Gata1 may suppress the activity of the human μLCR- β-globin promoter.
The aim of this study was to determine the effects of Gata1 on the activity of hGATA1 in mice. Since the Gata1null mutation is embryonically lethal, the hypothesis was tested by generating with standard genetic approaches double mutants carrying hGATA1 and the hypomorphic Gata1low mutation, a mutation that strongly reduces expression of Gata1 in adult hematopoietic cells (erythroblasts, MK, hematopoietic progenitors, others). If successful, these experiments would also indirectly assess the extent by which hGATA1 is capable to rescue the complex Gata1low phenotype. Gata1low mice, in fact, are born anemic and thrombocytopenic but recover from their anemia at 1 month by activating extramedullary hematopoiesis in spleen (Vannucchi et al Blood 2001;97:3040). Spleen erythropoiesis is however mostly ineffective since many of the erythroblasts present in this organ are in apoptosis (Tunel+). Gata1low mice remain thrombocytopenic all their life and with age develop myelofibrosis, a phenotype that resemble primary myelofibrosis, the most severe of the Phyladelphia-negative myeloproliferative neoplasms (Vannucchi et al Blood 2002;100:1123). In these mice, hematopoietic stem cells are detected in spleen, and not in bone marrow, and generate bipotent MK/erythroid progenitors (MEP) with a commitment program screwed toward the mast cell lineage and capable to generate in vitro at the single cell basis mast cell lines (Ghinassi et al. Blood 2007;109:1460-71).
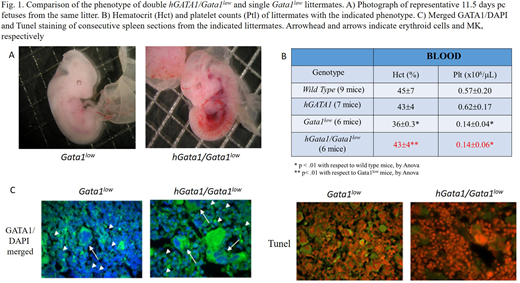
Double hGATA1Gata1low mutants were easily generated and were not anemic at birth. At 4 months, they expressed normal haematocrit but reduced blood platelet counts (Fig. 1A,B). RT-PCR analyses of prospectively isolated cell populations confermed that the transgene mRNA is barely detectable in cells from single hGATA1 mutants. By contrast, in the double mutants, hGATA1 mRNA was expressed by common myeloid progenitors, its expression increased by 10-fold in MEP, remained at the same levels expressed by MEP in erythroid cells (CD71+/Ter119+) but was barely detectable in MK (CD41+/CD61+). Expression of hGATA1 did not alter the levels of the endogenous Gata1 expressed by these populations that remained low.
MEP purified from double hGATA1/Gata1low mice generated in culture more cells (fold increase=250 in Gata1low/hGATA1 cultures vs 50 in single hGATA1 or Gata1low cultures) which were mostly composed by erythroid precursors unable to generate cell lines.
By contrast with single Gata1low mutants, by confocal microscopy, GATA1 was readily detectable in erythroid cells from bone marrow and spleen that these cells were not Tunel+ (Fig. 1C), indicating that hGATA1 reduced ineffective erythropoiesis.
GATA1 protein remained not detectable in MK from the same tissues (Fig. 1C) and these cells displayed the abnormal morphology characteristic of the retarded maturation of Gata1low MK. Furthermore, double hGATA1/Gata1low mice, as the single Gata1low ones, developed myelofibrosis in bone marrow (by Gomori staining) at 8 months, confirming that myelofibrosis is driven by MK abnormalities.
In conclusion, hGATA1 is expressed by adult CMP, MEP and erythroblasts in a genetic Gata1low background rescuing the MEP and erythroid phenotype induced by the hypomorphic mutation. hGATA1 is not expressed and does not rescue the MK phenotype of Gata1low mice, highlighting the specificity of the β-globin promoter for erythroid cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal